The average person will take more than 600 million breaths over the course of their life. Every breath stretches the lungs’ tissues with each inhale and relaxes them with each exhale. The mere motions of breathing are known to influence vital functions of the lungs, including the maintenance of healthy tissue.
Now, new research from the Wyss Institute at Harvard University has revealed that this constant pattern of stretching and relaxing does even more—it generates immune responses against invading viruses, such as COVID-19.
Using a ‘Human Lung Chip’ that replicates the structures and functions of the lung air sac, or “alveolus,” the research team discovered that by applying mechanical forces that mimic breathing motions, they could suppress influenza virus replication, while activating innate protective immune responses.
“This research demonstrates the importance of breathing motions for human lung function, including immune responses to infection, and shows that our Human Alveolus Chip can be used to model these responses in the deep portions of the lung, where infections are often more severe and lead to hospitalization and death,” said co-first author Haiqing Bai, Ph.D., a Wyss Technology Development Fellow at the Institute. The results were published this week in Nature Communications.
Creating a flu-on-a-chip
As the early phases of the COVID-19 pandemic made painfully clear, the lung is a vulnerable organ where inflammation, in response to infection, can generate a “cytokine storm” that can have deadly consequences. However, the lungs are also very complex, and it is difficult to replicate their unique features in the lab. This complexity has hindered science’s understanding of how the lungs function at the cell and tissue levels, in both healthy and diseased states.
RELATED: Researchers Find New Strategy for Preventing Clogged Arteries
The Wyss Institute’s Human Organ Chips were developed to address this problem, and have been shown to faithfully replicate the functions of many different human organs in the lab, including the lung. As part of projects funded by the NIH and DARPA since 2017, Wyss researchers have been working on replicating various diseases in Lung Airway and Alveolus Chips to study how lung tissues react to viruses that have pandemic potential, and test potential treatments.
During his Ph.D. training, Bai studied diseases that affect the tiny air sacs deep inside the lungs where oxygen is rapidly exchanged for carbon dioxide. That foundation prepared him to tackle the challenge of recreating a flu infection in an Alveolus Chip so that the team could study how these deep lung spaces mount immune responses against viral invaders.
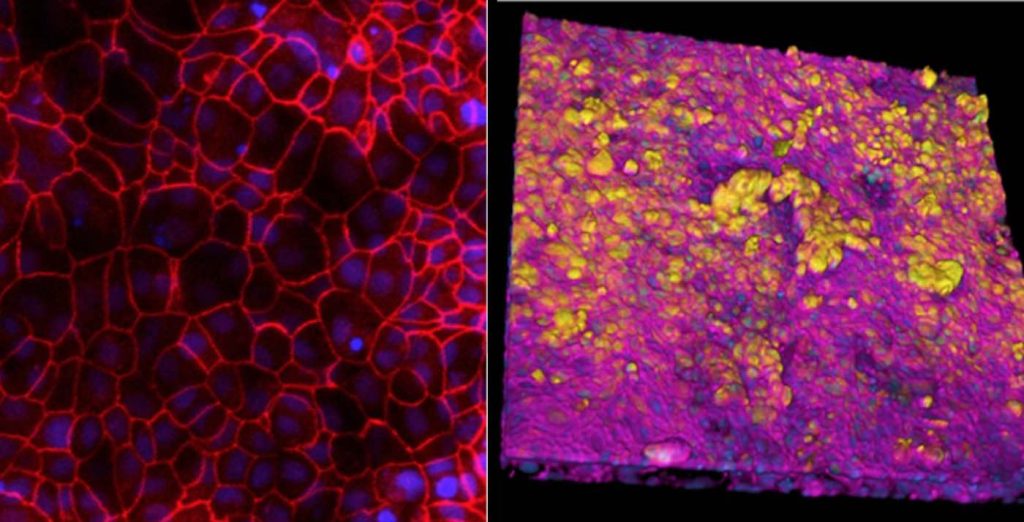
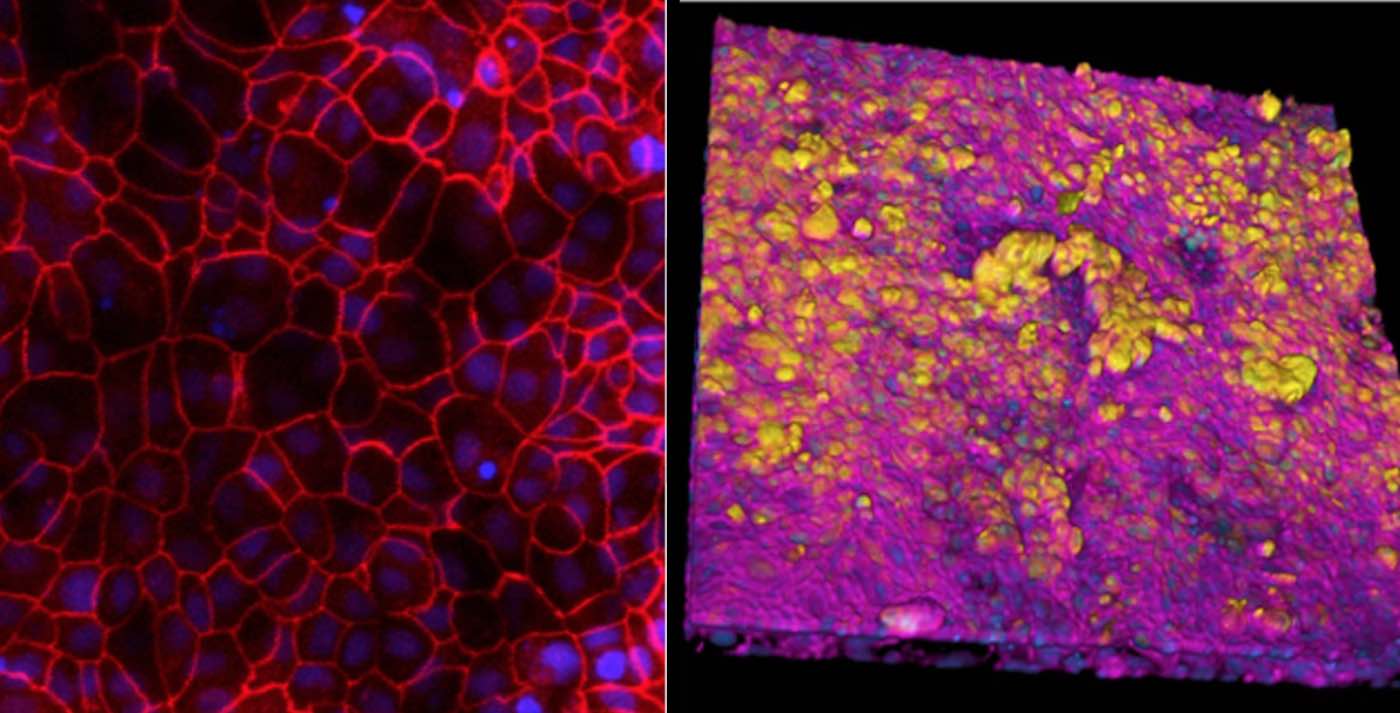
Bai and his team first lined the two parallel microfluidic channels of an Organ Chip with different types of living human cells – alveolar lung cells in the upper channel and lung blood vessel cells in the lower channel – to recreate the interface between human air sacs and their blood-transporting capillaries. To mimic the conditions that alveoli experience in the human lung, the channel lined by alveolar cells was filled with air while the blood vessel channel was perfused with a flowing culture medium containing nutrients that are normally delivered via the blood. The channels were separated by a porous membrane that allowed molecules to flow between them.
Previous studies at the Wyss Institute have established that applying cyclical stretching to Alveolus Chips to imitate breathing motions produces biological responses that mimic those observed in vivo. This is accomplished by applying suction to hollow side chambers adjacent to the cell-lined fluidic channels to rhythmically stretch and relax the lung tissues by 5%, which is what human lungs typically experience with every breath.
When the team infected these “breathing” Alveolus Chips with H3N2 influenza by introducing the virus into the air channel, they observed the development of several known hallmarks of influenza infection, including the breakdown of junctions between cells, a 25% increase in cell death, and the initiation of cellular repair programs. Infection also led to much higher levels of multiple inflammatory cytokines in the blood vessel channel including type III interferon, a natural defense against viral infection that is also activated in in vivo flu infection studies.
In addition, the blood vessel cells of infected chips expressed higher levels of adhesion molecules, which allowed immune cells including B cells, T cells, and monocytes in the perfusion medium to attach to the blood vessel walls to help combat the infection. These results confirmed that the Alveolus Chip was mounting an immune response against H3N2 that recapitulated what happens in the lung of human patients infected with flu virus.
Focus on your breath
The team then carried out the same experiment without mechanical breathing motions. To their surprise, chips exposed to breathing motions had 50% less viral mRNA in their alveolar channels and a significant reduction in inflammatory cytokine levels compared to static chips. Genetic analysis revealed that the mechanical strain had activated molecular pathways related to immune defense and multiple antiviral genes, and these activations were reversed when the cyclical stretching was stopped.
“This was our most unexpected finding – that mechanical stresses alone can generate an innate immune response in the lung,” said co-first author Longlong Si, Ph.D., a former Wyss Technology Development Fellow who is now a Professor at the Shenzhen Institute of Advanced Technology in China.
Knowing that sometimes the lungs experience greater than 5% strain, such as in chronic obstructive pulmonary disorder (COPD) or when patients are put on mechanical ventilators, the scientists increased the strain to 10% to see what would happen. The higher strain caused an increase in innate immune response genes and processes, including several inflammatory cytokines.
“Because the higher strain level resulted in greater cytokine production, it might explain why patients with lung conditions like COPD suffer from chronic inflammation, and why patients who are put on high-volume ventilators sometimes experience ventilator-induced lung injury,” Si explained.
The scientists then went a step further, comparing the RNA molecules present in cells within strained vs. static Alveolus Chips to see if they could pinpoint how the breathing motions were generating an immune response. They identified a calcium-binding protein, called S100A7, that was not detected in static chips but highly expressed in strained chips, suggesting that its production was induced by mechanical stretching. They also found that increased expression of S100A7 upregulated many other genes involved in the innate immune response, including multiple inflammatory cytokines.
Based on this promising result, the team then infected strained Alveolus Chips with the virus H3N2 and administered the drug azeliragon at its therapeutic dose two hours after infection.
This drug significantly blocked the production of inflammatory cytokines – an effect that was further enhanced when they added the antiviral drug molnupiravir (which was recently approved for patients with COVID-19) to the treatment regimen.
However, while azeliragon is a promising anti-inflammatory drug, the scientists warned that more studies are needed to determine a safe and effective treatment regimen in humans.
Meanwhile, robust breathing is something we can all do throughout any season to promote good heath.




















Fun fact: Yoga discovered that more than 4,700 years ago in India! It is really underrated, more people should learn about yoga.
Yeah! THANK YOU for mentioning Yoga. Can we once and for all embrace that it helps EVERYTHING? Try Kundalini, folks. It’s a miracle for all that ails us and can be done no matter what age, size or level of flexibility. Can even be done sitting in a wheelchair!