A single-dose gene replacement therapy is found to transform the capabilities for movement in children over 2 years of age and teenagers with spinal muscular atrophy, according to research published in Nature Medicine.
The effects allowed these minors who could sit but not stand to move like they’ve never done before, including standing up, walking, and even climbing stairs.
The real-world results of this phase 3 clinical trial, involving 126 children and adolescents, could support an alternative to lifelong, repeat-dose treatments for people who couldn’t get access to corrective treatment before 2, when curing the condition is possible.
Spinal muscular atrophy is a rare genetic condition that causes muscle weakness and loss of movement over time. It develops because the body cannot make enough of a protein, called survival motor neuron, needed for healthy nerve cells.
Onasemnogene abeparvovec is a gene therapy that restores production of this missing protein in a single treatment. However, it is currently approved in the US and Europe only as a single intravenous treatment for children under 2 years of age. Therefore, those older than 2 years of age can receive treatments only to slow the disease, and these must be taken regularly, either by injection or orally.
The financial burden for patients and their families is immense, with average 5-year inpatient costs of $116,000, and outpatient costs of $55,000. Around 9,000 people live with spinal muscular atrophy in the USA.
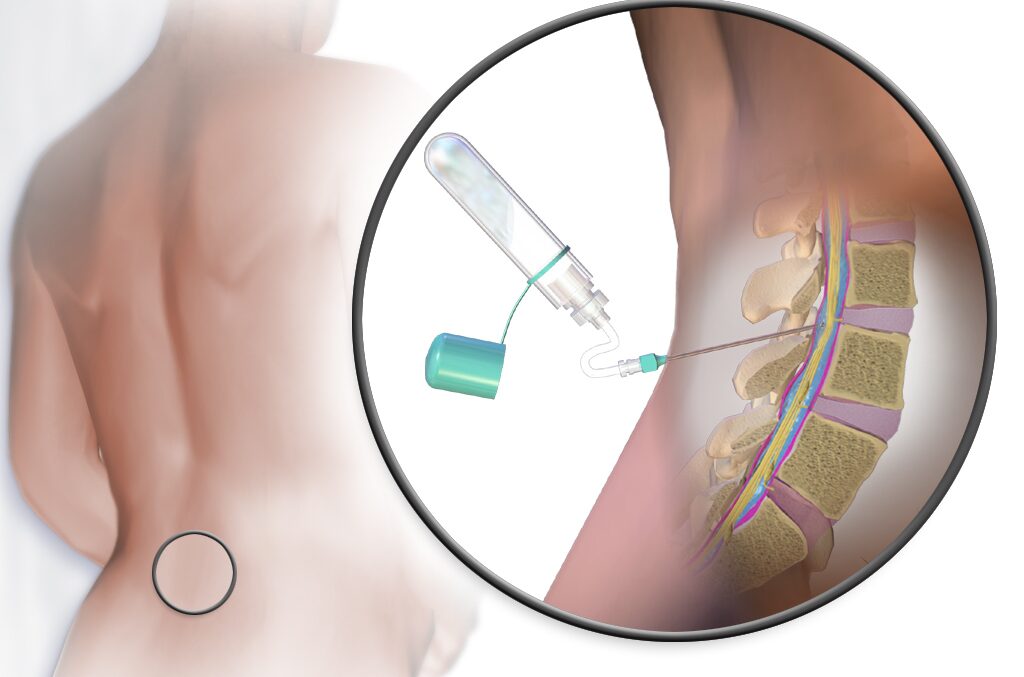
Lead author Richard Finkel at St. Jude Children’s Research Hospital in Tennessee and colleagues assessed the safety and efficacy of a single dose of the same gene therapy—onasemnogene abeparvovec delivered—directly into the spinal fluid of children older than 2 years of age with spinal muscular atrophy.
The year-long trial involved 126 children and adolescents between 2 and 18 years of age who were able to sit but had never walked on their own. The participants were randomly assigned to receive either the gene therapy (75 participants) or a placebo (51 participants). Those who received the active therapy achieved a significantly greater improvement in motor function scores on a validated test (which identified gains in 33 specific skills, such as moving from a lying into a sitting position, walking, and climbing stairs) compared with those who did not.
GENE THERPAIES TO THE RESCUE:
- Boy with Rare Genetic Disorder Amazes Doctors After World-First Gene Therapy
- UPDATE Long-Term Follow-up in Babies Born with ‘Bubble Boy Disease’ Still Seem Cured
- Always Fatal Huntington’s Disease is Successfully Treated for First Time With Gene Therapy
Side effects were similar in both groups and were generally manageable, and the only substantial weakness in the trial was that it lasted 12 months. Longer-term follow-ups would be necessary to establish safety and efficacy.
The findings suggest that the only treatment for spinal muscular atrophy is, in fact, effective in participants older than 2 years of age, but only when delivered directly into the spinal fluid.
Dr. Finkel and his colleagues recommend broadening access to this gene therapy for spinal muscular atrophy to patients beyond infancy, addressing an unmet need in older children and adolescents.
SHARE This Vital Advancement In The Treatment Of This Rare Disease…



















