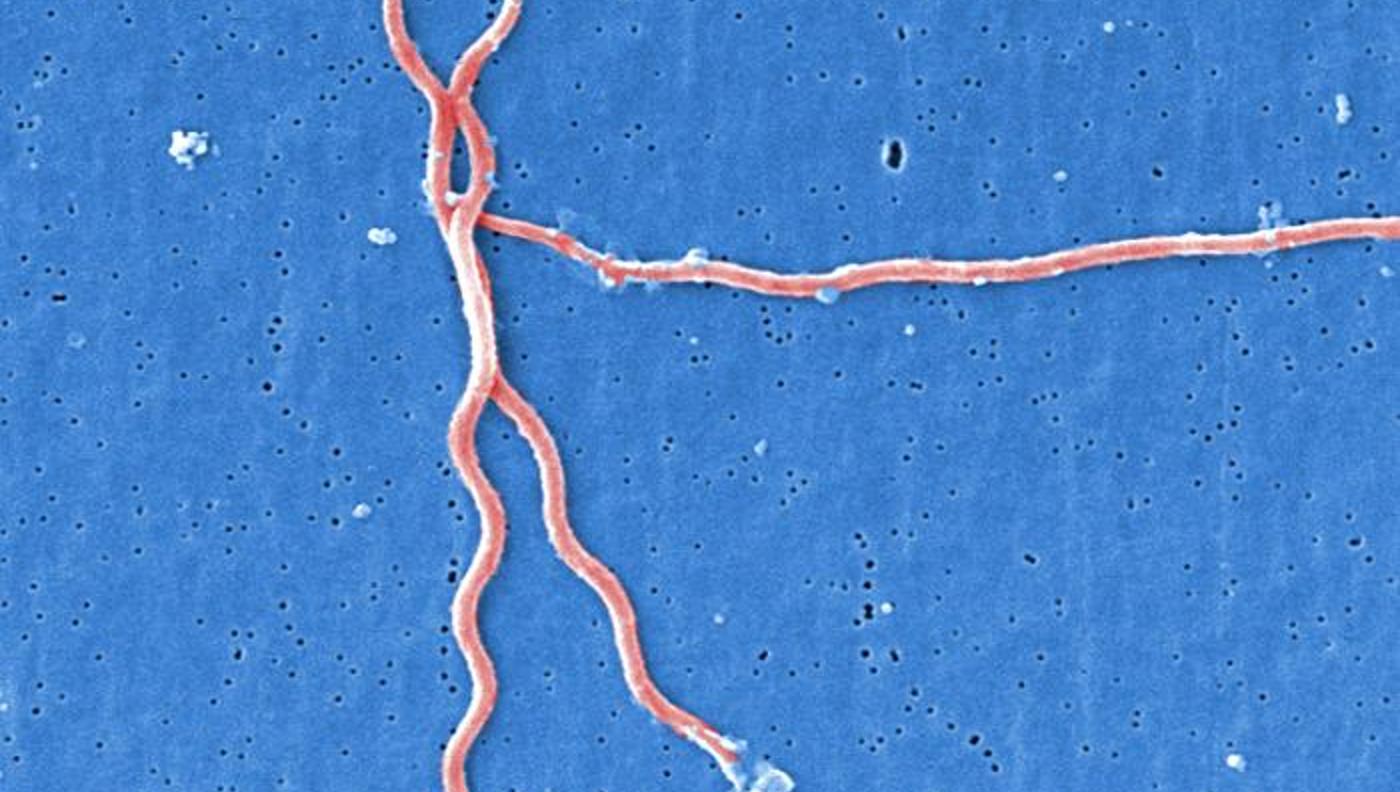
Researchers studying naturally occurring antibiotics have isolated one which eradicated the bacteria that causes Lyme disease, potentially offering a revolutionary treatment for the pathogen in both humans, and the natural environment.
Hygromycin A, which the scientists found in a screen of soil microbes, was found to clear the infection of B. burgdorferi, the bacteria found in tiny worm-like parasites which cause much of the worst effects of the disease, without harming the rest of the microbiome in live mice and human cells.
A debilitating disease that can put some people out of action for years, Lyme disease affects 500,000 Americans annually, costing an excess of $3 billion in medical care, and much more from lost labor hours. It drives a variety of pathologies, some minor, some major, and is treated principally by non-selective antibiotics which wreck havoc on the beneficial bacterial communities we all carry on our skin and in the gut.
After infecting mice with B. burgdorferi, hygromycin A, was administered twice a day for five days. Using a PCR test which stimulates rapid replication of even single cells, the treatment proved to clear every last trace of the infection.
Furthermore, in vitro tests on human cells found that even at completely unnecessarily-high doses, its therapeutic index was up there with some of the safest over-the-counter medicines.
Hygromycin A targets these worm-like spirochetes, which along with Lyme disease, also cause other diseases. Another spirochete cleared by hygromycin A is called T. pallidum, which not only causes syphilis, but has been a notable recipient of antibiotic resistance.
A super drug
Broad-spectrum antibiotics, along with killing our beneficial microbes, were shown in the paper to be strongly linked with blooms of harmful bacteria cropping up in the dead spaces left by an antibiotic like amoxicillin or doxycycline. They have also led to antibiotic resistance in many common pathogens.
MORE: U.S. Department of Defense Funds New Lyme Disease Vaccine Development
By contrast, hygromycin A treatment resulted in a rise of harmless species like lactobacillus.
“Lyme disease is on the rise and, in many locations, limits our ability to enjoy outdoor activities,” the authors write. “A more permanent solution would require eradicating the source of the disease. We show that baits containing hygromycin A clear B. burgdorferi infection in mice, the principal host of the pathogen.”
However the good news didn’t stop there for the team, as they began to reason that because hygromycin A selectively targeted spirochetes, the tiny parasites mentioned earlier, it could be possible to inoculate entire ecosystems against Lyme disease, since hygromycin A is also a naturally-occurring soil microbe, and therefore already has an established place in the terrestrial web of life.
They referenced a study which looked at how a parasite bait containing the common broad-spectrum doxycycline was spread across an area and eradicated the Lyme disease-causing pathogen in 87% of mice, and 94% of ticks.
“This is far above levels required to reduce infection below the… the percentage of infected ticks and mice needed to sustain the infectious cycle in the wild,” the authors note.
RELATED: Scientists Develop New Test That Can Diagnose Lyme Disease in Just 15 Minutes
“Doxycycline, however, is an essential part of our shrinking antibiotic arsenal, and spreading it on large territory is unfeasible due to the risk of selecting for resistant micro-organisms. Hygromycin A, with its limited activity against non-spirochetal organisms, would make an ideal reservoir-targeted antibiotic against B. burgdorferi.“
Not only could they be on a path of eliminating Lyme disease in humans, but perhaps in the natural environment as well, which along with numerous other enormous benefits, would allow people in prone areas to wear shorts and t-shirts on a summertime hike again, which merits some celebration.
CURE Negativity—This Story With Your Friends on Social Media…