Scientists at Johns Hopkins say that an experimental antibody drug appears to prevent and reverse the onset of type 1 diabetes in mice—and often lengthen their lives.
The drug called mAb43 is unique, according to the researchers, because it targets insulin-making beta cells in the pancreas directly and is designed to shield those cells from attacks by the body’s own immune system cells.
The drug’s specificity for such cells may enable long-term use in humans with few side effects, say the researchers. Such monoclonal antibodies are made by cloning, or making identical replicas of, an animal or human cell line.
The findings, reported in the May issue of Diabetes, raise the possibility of a new drug for type 1 diabetes, an autoimmune condition that affects about 2 million American children and adults and has no cure or means of prevention.
Unlike type 2 diabetes, in which the pancreas makes too little insulin, in type 1 diabetes, the pancreas makes no insulin because the immune system attacks the pancreatic cells that make it, cutting off the body’s ability to regulate blood sugar levels.
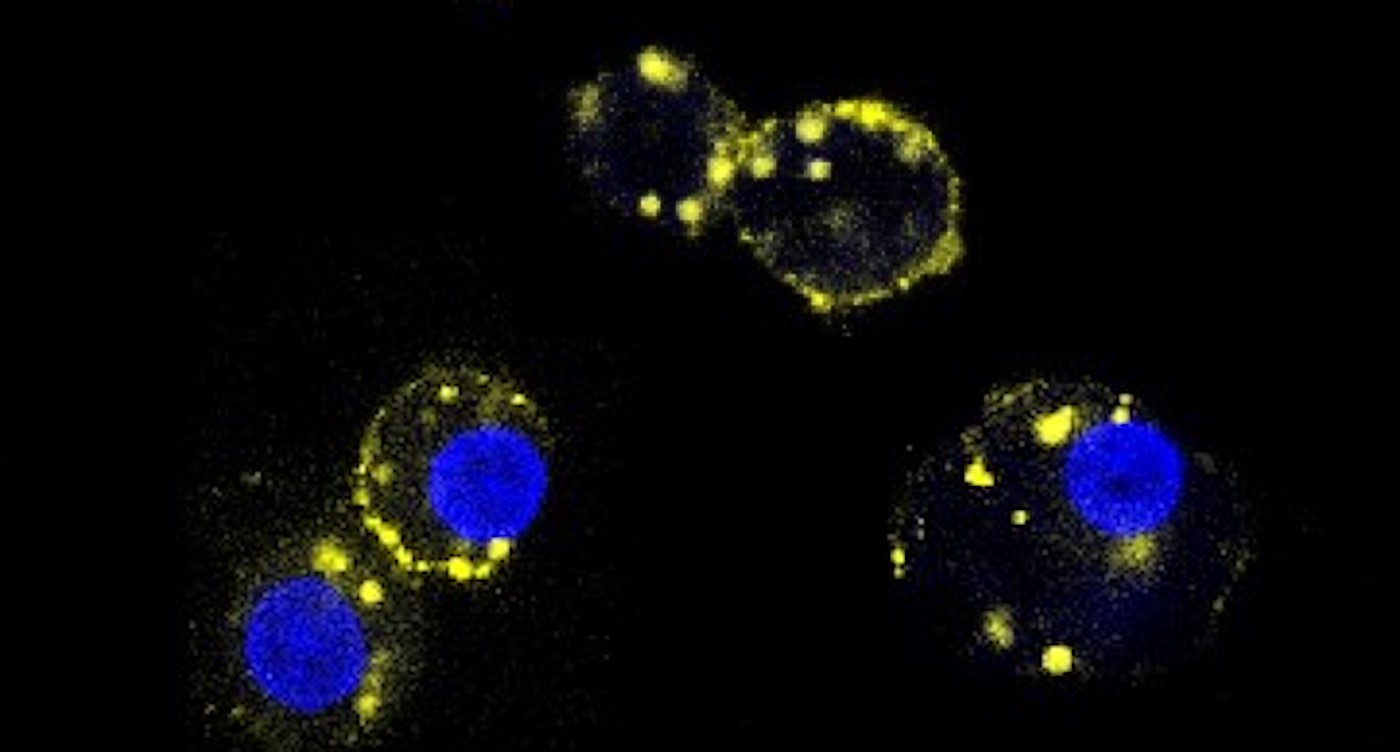
Dax Fu, Ph.D., an associate professor of physiology at the Johns Hopkins University School of Medicine and leader of the research team, says mAb43 binds to a small protein on the surface of beta cells, which dwell in clusters called islets. The drug was designed to provide a kind of shield or cloak to hide beta cells from immune system cells that attack them as “invaders.”
The researchers used a mouse version of the monoclonal antibody, and will need to develop a humanized version for studies in people.
64 non-obese mice bred to develop type 1 diabetes were given a weekly dose of mAb43 via intravenous injection when they were 10 weeks old. After 35 weeks, all mice were non-diabetic.
BEAT DIABETES: Dancing or Brisk Walks Can Slash Diabetes Risk By 74%, Even When it Runs in the Family
In five of the same type of diabetes-prone mice, the researchers held off giving weekly mAb43 doses until they were 14 weeks old, and then continued dosages and monitoring for up to 75 weeks. “One of the five in the group developed diabetes, but no adverse events were found,” say the researchers.
When mAb43 was given early on, the mice lived much longer, for the duration of the monitoring period over 75 weeks. Comparatively, the control group of mice that did not receive the drug lived only 18–40 weeks.
Next, the researchers, funded in part by the National Institutes of Health, looked more closely at the mice that received mAb43 and used a biological marker called Ki67 to see if beta cells were multiplying in the pancreas. After treatment with the antibody, immune cells retreated from beta cells, reducing the amount of inflammation in the area. In addition, beta cells slowly began reproducing.
“mAb43 in combination with insulin therapy may have the potential to gradually reduce insulin use while beta cells regenerate, ultimately eliminating the need to use insulin supplementation for glycemic control,” says team member and postdoctoral fellow Devi Kasinathan.
ANOTHER BREAKTHROUGH: New Artificial Pancreas for Type 2 Diabetes Manages Blood Sugar Twice as Well as Jabs –Now Approved in UK
The research team found that mAb43 specifically bound to beta cells, which make up about 1% or 2% of pancreas cells.
Another monoclonal antibody drug, teplizumab, was approved by the U.S. Food and Drug Administration in 2022. It binds to T cells, making them less harmful to insulin-producing beta cells. The drug has been shown to delay the onset of clinical (stage 3) type 1 diabetes by about two years, giving young children who get the disease time to mature and learn to manage lifelong insulin injections and dietary restrictions.
“It’s possible that mAb43 could be used for longer than teplizumab and delay diabetes onset for a much longer time, potentially for as long as it’s administered,” says Fu.
GREAT NEWS: Insulin Prices Now a Thing of the Past in U.S. After Government Initiates Monthly Cost of $35
In an ongoing effort, the Hopkins researchers aim to develop a humanized version of the antibody and conduct clinical trials to test for side effects and its ability to prevent type 1 diabetes altogether.
(Source: Hopkins Medicine)
HAIL THE HOPE By Sharing the Research on Social Media…