In South Korea, chemists have recently developed a way to grow artificial micro-diamonds in minutes, rather than days.
Furthermore, the technique doesn’t require high temperatures or intense pressure, and are made “from scratch” with the potential to revolutionize the diamond industry by providing unlimited micro-diamonds for polishing and cutting uses.
Gemstones are formed typically by intense heat, intense pressure, natural catalysts, or some combination of the three. Diamonds require an awful lot of the first two to manufacture artificially, but Rodney Ruoff, a physical chemist at the Institute for Basic Science in South Korea, has eliminated the need.
Instead of gigapascals of pressure and temperatures as high as 2,700 degrees Fahrenheit (1,500 degrees Celsius), Ruoff and his colleagues needed graphene, silicon, gallium, iron, and nickel—and that’s it.
“For over a decade I have been thinking about new ways to grow diamonds, as I thought it might be possible to achieve this in what might be unexpected (per ‘conventional’ thinking) ways,” Ruoff told Live Science by email.
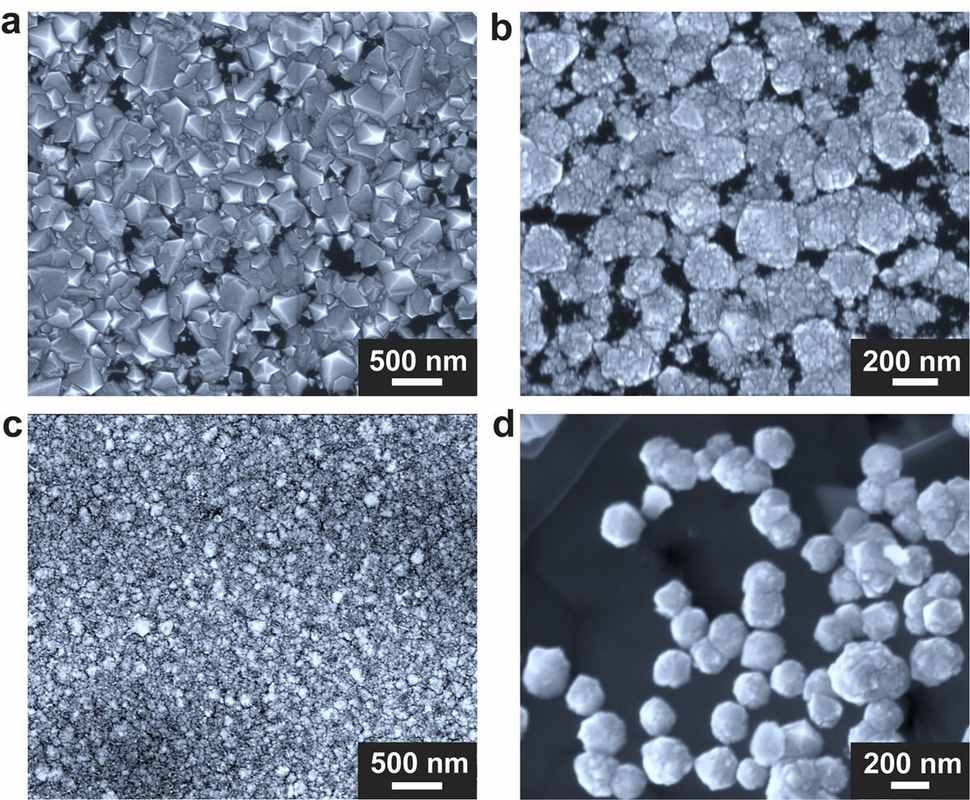
Ruoff started with gallium, which had been shown in a previous, unrelated paper to catalyze the formation of graphene. Graphene is pure carbon, just like a diamond, but the chemical structure is straight and flat rather than the latter’s tetrahedral formation.
At that point, the experiment met its most expensive component—a home-built chamber designed by co-author Won Kyung Seong—that could house the gallium-nickel-iron mixture in a graphene crucible and withstand the introduction of hot methane gas. When all these elements came together—with a pinch of silicon—diamonds were formed in 15 minutes at sea level pressures inside the chamber.
MORE DIAMOND SCIENCE: Huge Black Diamond Sold for $4.3 Million–and No One Knows Where it Came From or How it Was Formed
The chemical minutiae of how the experiment succeeded is still murky, and it will be at least another few years before the scientists or others working with similar methods will know for sure whether Ruoff’s process can produce diamonds for jewelry, because the ones described in their study were thousands of times smaller than lab-grown diamonds used for jewelry.
CAN YOU BELIEVE THIS: Rare Diamond Within a Diamond Is Unearthed in India and Dubbed ‘The Beating Heart’
However, the ‘film’ of micro-diamonds could very well serve to take the place of larger diamonds for the purpose of being crushed into powder. Because diamond is the hardest substance known, diamond dust is the tool by which diamonds are polished, and Ruoff’s 15-minute micro-diamonds (copyright available) may save jewelers thousands of dollars in the cost of diamond dust.
SHARE This Awesome Experimental Chemistry With Your Friends…



















