A legitimate quality-of-life enhancing treatment is now available for a class of patient for whom treatments come few and far between.
These patients are sometimes called “butterfly children,” as they are born with a disease that prevents their skin cells from coding certain proteins, resulting in a skin organ so delicate that the slightest touch can cause it to rupture and blister for months.
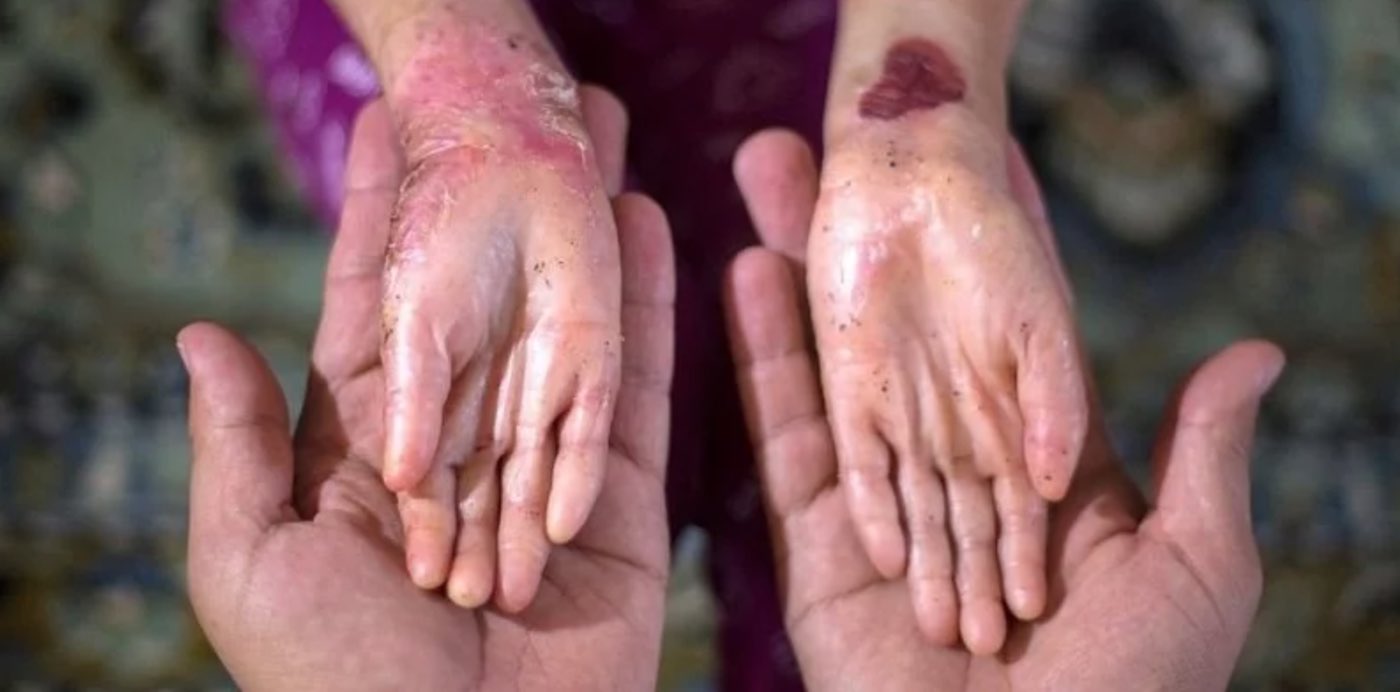
A DNA-coding skin cell represents a genetic therapy option for those suffering from this disease, known scientifically as epidermolysis bullosa (EB). When a few drops were applied onto a wound, which under normal conditions of EB might never heal, a trial in nine human patients found substantial improvement.
The gel is known as B-VEC, and consists of the non-replicating shell of a Herpes Type 1 virus that’s been engineered to carry the genetic codes to make a protein called collagen VII. The lack of a strong fibral anchor—or component for anchoring different layers of the skin and internal organs together—resulting from an inability to produce collagen VII is the primary mutation that causes EB.
In phase 1 and 2 trials on mice and then on humans, goals for wound surface area reduction, time to wound closure, and duration of wound closure post treatment following B-VEC application were all met, and the parent pharmaceutical company, Krystal Biotech, is now seeking to jump Phase III trials since efficacy was already demonstrated. They are seeking regulatory approval directly to ensure the gel can be available for suffering children as soon as possible.
New hope
No side effects in the trials were reported. Herpes is extremely difficult to detect, which makes it an excellent vector for gene therapies, as host immune responses are very rarely triggered. While it seems strange to heap praise on Herpes, it’s not only difficult to detect, but one of the only genetic therapy deliver vectors that can hold the large biological computer file that codes for collagen VII.
MORE: Nigerian Mom Designs Solar-Powered Cribs That Put an End to Baby Jaundice Disease
The gel had to be spread by a bandage to prevent further skin damage, and the patients were treated every 1 to 3 days, for 25 days. In all but one patient, the wounds were healed 3 months after treatment, and didn’t reopen. Compared to placebo, the wounds healed better and stayed shut longer.
RELATED: Self-Compassion Is Actually Good for Your Heart Health
“It’s not a permanent cure, but it’s a way to really keep on top of the wounds,” study lead and Director of the Blistering Disease Clinic at Stanford Health Care Dr. Peter Marinkovich said in a statement. “It significantly improves patients’ quality of life.”
Since the phase II results were published, Markinovich also revealed the positive results of second, larger trial of the gel at the 2022 American Academy of Dermatology Annual Meeting in Boston, Mass.
Since EB is not limited to the skin, but also affects internal organs subject to friction against others like the esophagus, corneas, and anus, Markinovich is launching a trial to test an endogenous form of the gel to treat these injuries while Krystal seeks approval from the FDA.
SHARE the Delicate Wings of Hope; Share This Story…