Chronic inflammation can contribute to a variety of devastating diseases, from Alzheimer’s and Parkinson’s to diabetes and cancer. It flares up if old age, stress, or environmental toxins keep the body’s immune system in overdrive.
Now, scientists at the University of California, Berkeley, have identified a molecular “switch” that controls the immune machinery responsible for chronic inflammation in the body. The results of their testing on mice could lead to new ways to halt or even reverse many of these age-related conditions.
“My lab is very interested in understanding the reversibility of aging,” said senior author Danica Chen, associate professor of metabolic biology, nutritional sciences and toxicology at UC Berkeley. “In the past, we showed that aged stem cells can be rejuvenated. Now, we are asking: to what extent can aging be reversed? And we are doing that by looking at physiological conditions, like inflammation and insulin resistance, that have been associated with aging-related degeneration and diseases.”
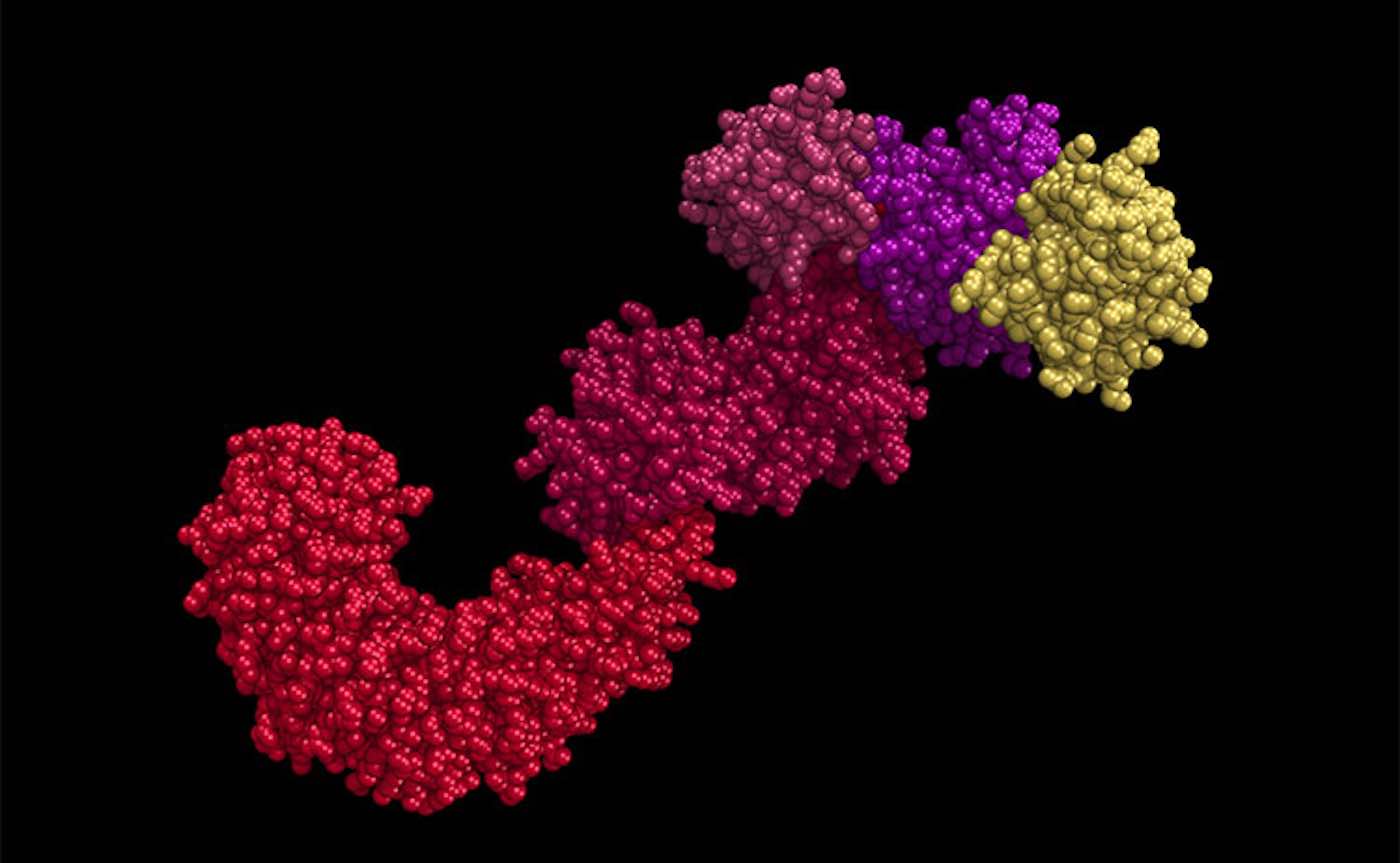
In the study, published online in the journal Cell Metabolism this month, Chen and her team show that a bulky collection of immune proteins called the NLRP3 inflammasome—responsible for sensing potential threats to the body and launching an inflammation response—can be essentially switched off by removing a small bit of molecular matter in a process called deacetylation.
Over-activation of the NLRP3 inflammasome has been linked to a variety of chronic conditions, including multiple sclerosis, cancer, diabetes, and dementia. Chen’s results suggest that drugs targeted toward deacetylating—or switching off—this NLRP3 inflammasome might help prevent or treat these conditions and possibly age-related degeneration in general.
“This acetylation can serve as a switch,” Chen said. “So, when it is acetylated, this inflammasome is on. When it is deacetylated, the inflammasome is off.”
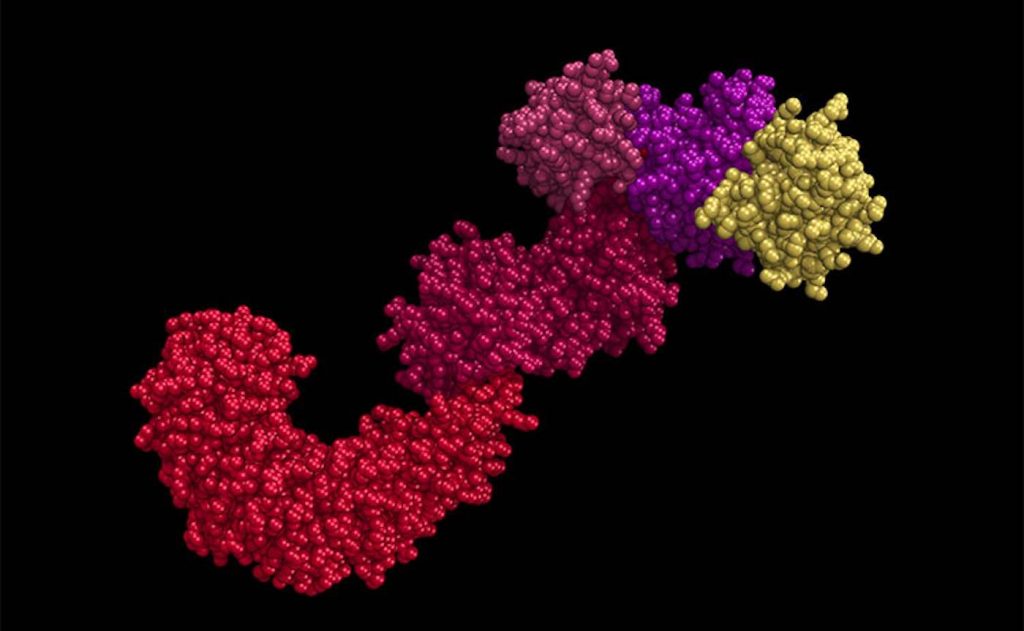
By studying mice and immune cells called macrophages, the team found that a protein called SIRT2 is responsible for deacetylating the NLRP3 inflammasome. Mice that were bred with a genetic mutation that prevented them from producing SIRT2 showed more signs of inflammation at the ripe old age of two than their normal counterparts. These mice also exhibited higher insulin resistance, a condition associated with type 2 diabetes and metabolic syndrome.
The team also studied older mice whose immune systems had been destroyed with radiation and then reconstituted with blood stem cells that produced either the deacetylated or the acetylated version of the NLRP3 inflammasome.
Those that were given the deacetylated, or “off”, version of the inflammasome had improved insulin resistance after six weeks, indicating that switching off this immune machinery might actually reverse the course of metabolic disease.
MORE: Accidental Discovery of New T-Cell Hailed as Major Breakthrough for ‘Universal’ Cancer Therapy
This is just one of the most recent discoveries of how neurological circuits can impact everything from overeating and alcoholism to celiac’s disease and epilepsy, although this trailblazing piece of research does shed further light on how inflammation can dramatically affect neurological conditions.
“I think this finding has very important implications in treating major human chronic diseases,” Chen said. “It’s also a timely question to ask, because in the past year, many promising Alzheimer’s disease trials ended in failure. One possible explanation is that treatment starts too late, and it has gone to the point of no return. So, I think it’s more urgent than ever to understand the reversibility of aging-related conditions and use that knowledge to aid a drug development for aging-related diseases.”
Reprinted from University of California Berkeley
Treat Your Friends To The Good News By Sharing It To Social Media…