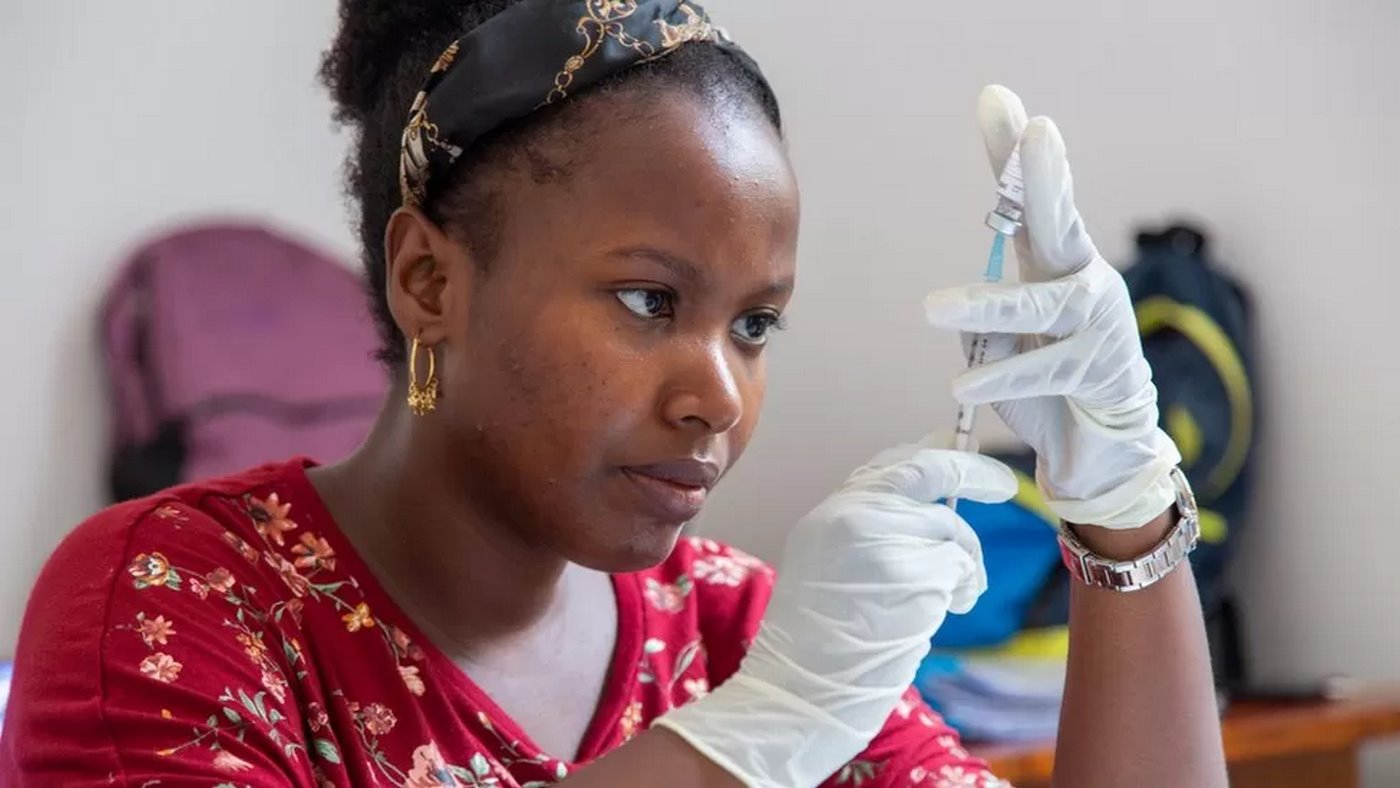
A new vaccine from Oxford, called R21, has been shown to be not only effective for the prevention of malaria in children, but also easier to make with a lower price tag—so will help protect a greater number of African countries.
The World Health Organization (WHO) officially recommended the R21/Matrix-M vaccine this week—the second malaria vaccine in two years, following the RTS,S/AS01 vaccine, which received a WHO recommendation in 2021.
Both vaccines are shown to be safe and effective in preventing malaria in children, the deadly disease that killed 619,000 people in 2021 alone.
When implemented broadly, the treatments are expected to have a major public health impact against the mosquito-borne disease, particularly in the African Region.
Demand for the malaria vaccines is high, but, available supply of RTS,S is limited. The addition of R21 will boost supply that could benefit all children living in these areas.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” said Dr. Tedros Adhanom Ghebreyesus, WHO Director-General. “Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster, and to bring us closer to our vision of a malaria-free future.”
The WHO Regional Director for Africa says the second vaccine holds real potential to close the supply gap for hundreds of thousands of young lives in Africa.
The ongoing R21 vaccine clinical trial and other studies showed:
- High efficacy when given just before the high transmission season: In areas with highly seasonal malaria transmission (contained to 4-5 months per year), the R21 vaccine was shown to reduce symptomatic cases of malaria by 75% during the 12 months following a 3-dose series.
- A fourth dose given a year after the third maintained the vaccine’s efficacy. This high efficacy is similar to that demonstrated when RTS,S is given seasonally.
- Good efficacy when given in an age-based schedule: The vaccine showed good efficacy (66%) during the 12 months following the first 3 doses. A fourth dose one year after the third maintained efficacy.
- Cost effectiveness: R21 costs just $2-4 per dose, compared to RTS,S, sold under the name of Mosquirix by London pharmaceutical company GSK, which costs around $9.80 per dose, according to Nature.
- Safety: The R21 vaccine was shown to be safe in clinical trials; and safety monitoring will continue.
The two WHO-recommended vaccines have not been tested in a head-to-head trial to compare performance, so the choice of product to be used in each country will be based on programmatic characteristics, vaccine supply, and affordability
At least 28 African countries plan to introduce one of the malaria vaccines as part of their national immunization programs—with the Vaccine Alliance Gavi providing technical and financial support to this effort in 18 countries. The RTS,S vaccine will be rolled out in some African countries in early 2024, with R21 becoming available to countries in the middle of next year.
MORE GOOD HEALTH NEWS: After Taking Vitamin B2 Baby Becomes Solitary Case of Recovery from Rare Genetic Disease
MULTIPLY The GREAT News For Africa By Sharing on Social Media…